- Clarity and confidence
-
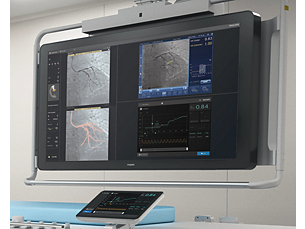
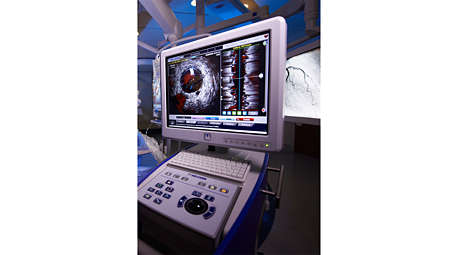
One system, many choices
Core Mobile supports a full suite of imaging and physiology analysis tools including FFR lesion assessment, iFR modality, iFR Scout, digital IVUS, high resolution rotational IVUS, ChromaFlo stent apposition assessment, VH IVUS automatic tissue classification, and Pioneer Plus IVUS for peripheral procedures. - iFR modality
-
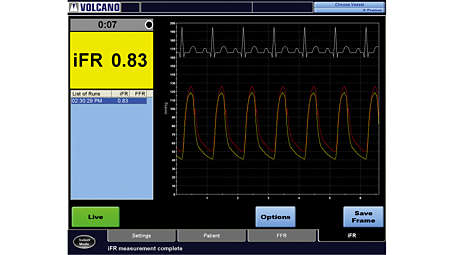
iFR modality simplifies workflow
iFR modality simplifies workflow by providing a hyperemia-free measurement to assess lesion significance in as few as five heartbeats. Philips’ proprietary iFR modality has a robust body of clinical evidence with over 9,000 patients in numerous studies and peer-reviewed journal articles.⁵ - iFR Scout pullback
-
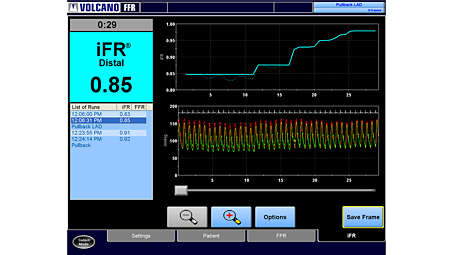
iFR Scout pullback
The iFR Scout pullback shows the most significant gradient in the mid-vessel lesion with diffuse proximal disease. - Fractional Flow Reserve
-
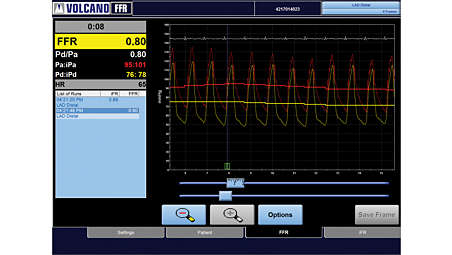
Fractional Flow Reserve measurement
Various clinical studies demonstrate that physiologic lesion assessment by FFR to guide routine PCI is superior to current angiography guided treatment. This measured ratio represents the potential decrease in coronary flow distal to the coronary stenosis.⁶ - IVUS assesses disease
-
IVUS helps with disease assessment
IVUS imaging helps physicians assess disease markers, including plaque burden percentage, lesion location and morphology, calcium volume, and the presence of thrombus. It also provides analysis of crucial parameters—like luminal cross-sectional measurements—and helps aid in disease diagnosis. - VH IVUS
-
Real-time lesion assessment in the cath lab
VH IVUS Imaging provides a colorized tissue map of plaque composition with automated lumen and vessel measurements. VH IVUS technology uses advanced, proprietary spectral analysis techniques to classify plaque into 4 tissue types with 93-97% accuracy.⁹ - Grayscale
-
Grayscale enhances procedures
Grayscale enhances angiography procedures by enabling detailed views. Angiography produces a shadowgram of contrast, while IVUS visualizes extent and location of plaque, enabling precise disease assessment, vessel, and optimal stent placement. IVUS guidance has been associated with a 74% change in PCI strategy, and reduced MACE, MI, TLR, and death in large studies.⁷,⁸ - ChromaFlo
-
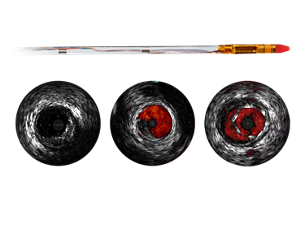
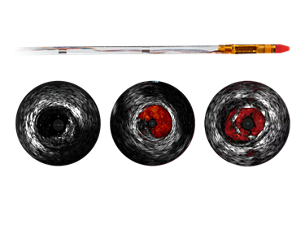
ChromaFlo stent apposition assessment
ChromaFlo highlights blood flow red for easy assessment of stent apposition, lumen size, and more. Appropriate for peripheral and coronary vessels, including left main, bifurcations, superficial femoral artery and iliac. It is designed to make lumen size and stent apposition instantly recognizable and helps identify branches, dissections, and plaque in bifurcations. - Convenience
-
True integration and convenience
Core Mobile delivers convenience by offering flexibility to service multiple rooms. Only Philips offers the plug-and-play simplicity of digital IVUS, touchscreen control from the sterile field to get to your answers faster, and the hyperemia-free iFR modality.²,³ - Optimal ease of use
-
Intuitive interface
Core Mobile delivers an intuitive interface for optimal ease of use as well as guided workflows and uniform controls to simplify training. Convenient measurements and labeling tools are available to document findings. Core Mobile offers a streamlined workflow with DICOM Worklist for patient data transfer. You can archive your results via DICOM Store, DVD, or printout.²,⁴
One system, many choices
One system, many choices

One system, many choices
iFR modality simplifies workflow
iFR modality simplifies workflow

iFR modality simplifies workflow
iFR Scout pullback
iFR Scout pullback

iFR Scout pullback
Fractional Flow Reserve measurement
Fractional Flow Reserve measurement

Fractional Flow Reserve measurement
IVUS helps with disease assessment
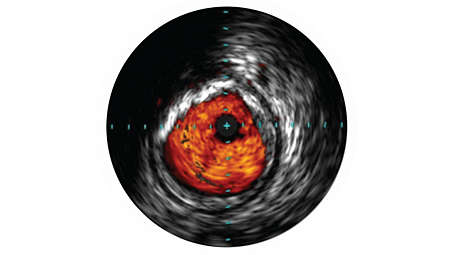
IVUS helps with disease assessment

IVUS helps with disease assessment
Real-time lesion assessment in the cath lab
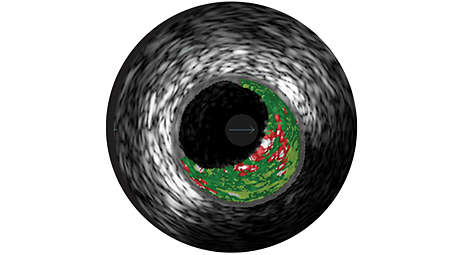
Real-time lesion assessment in the cath lab

Real-time lesion assessment in the cath lab
Grayscale enhances procedures
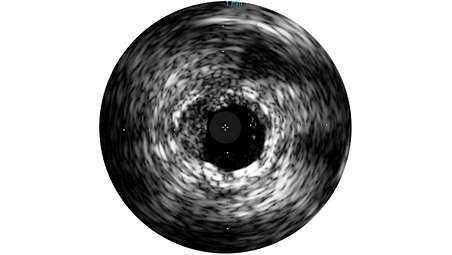
Grayscale enhances procedures

Grayscale enhances procedures
ChromaFlo stent apposition assessment
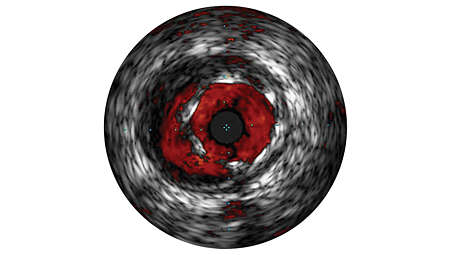
ChromaFlo stent apposition assessment

ChromaFlo stent apposition assessment
True integration and convenience

True integration and convenience

True integration and convenience
Intuitive interface
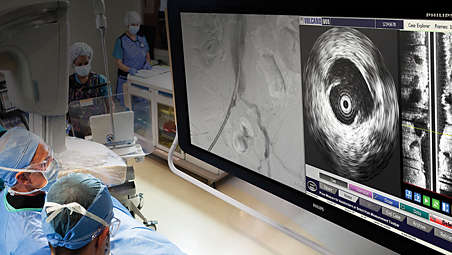
Intuitive interface

Intuitive interface
- Clarity and confidence
- iFR modality
- iFR Scout pullback
- Fractional Flow Reserve
- Clarity and confidence
-
One system, many choices
Core Mobile supports a full suite of imaging and physiology analysis tools including FFR lesion assessment, iFR modality, iFR Scout, digital IVUS, high resolution rotational IVUS, ChromaFlo stent apposition assessment, VH IVUS automatic tissue classification, and Pioneer Plus IVUS for peripheral procedures. - iFR modality
-
iFR modality simplifies workflow
iFR modality simplifies workflow by providing a hyperemia-free measurement to assess lesion significance in as few as five heartbeats. Philips’ proprietary iFR modality has a robust body of clinical evidence with over 9,000 patients in numerous studies and peer-reviewed journal articles.⁵ - iFR Scout pullback
-
iFR Scout pullback
The iFR Scout pullback shows the most significant gradient in the mid-vessel lesion with diffuse proximal disease. - Fractional Flow Reserve
-
Fractional Flow Reserve measurement
Various clinical studies demonstrate that physiologic lesion assessment by FFR to guide routine PCI is superior to current angiography guided treatment. This measured ratio represents the potential decrease in coronary flow distal to the coronary stenosis.⁶ - IVUS assesses disease
-
IVUS helps with disease assessment
IVUS imaging helps physicians assess disease markers, including plaque burden percentage, lesion location and morphology, calcium volume, and the presence of thrombus. It also provides analysis of crucial parameters—like luminal cross-sectional measurements—and helps aid in disease diagnosis. - VH IVUS
-
Real-time lesion assessment in the cath lab
VH IVUS Imaging provides a colorized tissue map of plaque composition with automated lumen and vessel measurements. VH IVUS technology uses advanced, proprietary spectral analysis techniques to classify plaque into 4 tissue types with 93-97% accuracy.⁹ - Grayscale
-
Grayscale enhances procedures
Grayscale enhances angiography procedures by enabling detailed views. Angiography produces a shadowgram of contrast, while IVUS visualizes extent and location of plaque, enabling precise disease assessment, vessel, and optimal stent placement. IVUS guidance has been associated with a 74% change in PCI strategy, and reduced MACE, MI, TLR, and death in large studies.⁷,⁸ - ChromaFlo
-
ChromaFlo stent apposition assessment
ChromaFlo highlights blood flow red for easy assessment of stent apposition, lumen size, and more. Appropriate for peripheral and coronary vessels, including left main, bifurcations, superficial femoral artery and iliac. It is designed to make lumen size and stent apposition instantly recognizable and helps identify branches, dissections, and plaque in bifurcations. - Convenience
-
True integration and convenience
Core Mobile delivers convenience by offering flexibility to service multiple rooms. Only Philips offers the plug-and-play simplicity of digital IVUS, touchscreen control from the sterile field to get to your answers faster, and the hyperemia-free iFR modality.²,³ - Optimal ease of use
-
Intuitive interface
Core Mobile delivers an intuitive interface for optimal ease of use as well as guided workflows and uniform controls to simplify training. Convenient measurements and labeling tools are available to document findings. Core Mobile offers a streamlined workflow with DICOM Worklist for patient data transfer. You can archive your results via DICOM Store, DVD, or printout.²,⁴
One system, many choices

One system, many choices

One system, many choices
iFR modality simplifies workflow

iFR modality simplifies workflow

iFR modality simplifies workflow
iFR Scout pullback

iFR Scout pullback

iFR Scout pullback
Fractional Flow Reserve measurement

Fractional Flow Reserve measurement

Fractional Flow Reserve measurement
IVUS helps with disease assessment

IVUS helps with disease assessment

IVUS helps with disease assessment
Real-time lesion assessment in the cath lab

Real-time lesion assessment in the cath lab

Real-time lesion assessment in the cath lab
Grayscale enhances procedures

Grayscale enhances procedures

Grayscale enhances procedures
ChromaFlo stent apposition assessment

ChromaFlo stent apposition assessment

ChromaFlo stent apposition assessment
True integration and convenience

True integration and convenience

True integration and convenience
Intuitive interface

Intuitive interface

Intuitive interface
Documentation
-
Brochure (1)
-
Brochure
- Product brochure (2.1 MB)
-
Brochure (1)
-
Brochure
- Product brochure (2.1 MB)
-
Brochure (1)
-
Brochure
- Product brochure (2.1 MB)
Specifications
- Power requirements
-
Power requirements System input - 100, 120V or 240VAC, 50/60Hz, 1000VA
-
- Dimensions
-
Dimensions Core Mobile - H=62", W=22", D=33" Inches
Control Pad (optional) - H=2.75", W=10.5", D=8.3" Inches
-
- Ordering information
-
Ordering information Core Mobile 120V - COREmb120
Control Pad - CPADO1
Bedrail mount - MNTO1
Monitor mount - MNTO2
-
- Power requirements
-
Power requirements System input - 100, 120V or 240VAC, 50/60Hz, 1000VA
-
- Dimensions
-
Dimensions Core Mobile - H=62", W=22", D=33" Inches
Control Pad (optional) - H=2.75", W=10.5", D=8.3" Inches
-
- Power requirements
-
Power requirements System input - 100, 120V or 240VAC, 50/60Hz, 1000VA
-
- Dimensions
-
Dimensions Core Mobile - H=62", W=22", D=33" Inches
Control Pad (optional) - H=2.75", W=10.5", D=8.3" Inches
-
- Ordering information
-
Ordering information Core Mobile 120V - COREmb120
Control Pad - CPADO1
Bedrail mount - MNTO1
Monitor mount - MNTO2
-
Related products
Alternative products
-
SyncVision
- iFR Co-registration
- IVUS Co-registration
- iFR and IVUS Tri-Registration
- Calibrated QCA
- Vessel enhancement
View product
-
Eagle Eye Platinum
- #1 choice of physicians for intravascular imaging (in the US).*
- GlyDx hydrophilic coating for increased lubricity
- A long, rapid exchange lumen for improved pushability
- Three radiopaque markers
- Compatibility with SyncVision for co-registration with angiography
View product
-
Eagle Eye Platinum ST
- A 2.5 mm tip-to-imaging distance designed to assess more of the vessel than standard catheters
- Plug-and-play simplicity
- Three radiopaque markers
- GlyDx hydrophilic coating
- SyncVision compatibility
View product
-
Verrata Plus
- New in-line clip connector for improved work flow
- New high fidelity sensor for reliable pressure measurement
- Reliably connect and disconnect with confidence
- Seamlessly switch between FFR and iFR, the only resting index with over 4500 patients studied
View product
-
SyncVision
The SyncVision precision guidance system is suitable with IntraSight and Core Integrated interventional platforms and streamlines lesion assessment, simplifies vessel sizing and enables precise therapy delivery all while integrating seamlessly in daily workflows in interventional suites of choice.
View product
-
Eagle Eye Platinum
The Eagle Eye Platinum digital IVUS catheter is the #1 choice of physicians for intravascular imaging (in the US).* As a unique plug-and-play intravascular imaging catheter it is designed for ease of use and deliverability. Features include a soft tapered tip, GlyDx hydrophilic coating for increased lubricity, a long, rapid exchange lumen for improved pushability, three radiopaque markers, and compatibility with SyncVision for co-registration with angiography.
View product
-
Eagle Eye Platinum ST
The Eagle Eye Platinum ST digital IVUS catheter offers a 2.5 mm tip-to-imaging distance designed to assess more of the vessel than standard catheters by providing a closer visualization of highly stenosed lesions and distal anatomy. The short tip catheter fits through all 5F guides and has all features of our top-selling Eagle Eye Platinum model, including plug-and-play simplicity, three radiopaque markers, GlyDx hydrophilic coating, and SyncVision compatibility.*
View product
See all related products
- 1. 505-0100.21, Operator's Manual, Core Integrated, 3.4X (p. 18); 505-0101.16, Operator's Manual, s5 Series FFR_iFR Option, v3.4x (p.15).
- 2. VAL RPT, S5-Core V3.4 SW with Core Control Pad, 215-0007.02.
- 3. Product Spec. 809480-001, 202-0407.01.
- 4. Requirements Specification Meridian VH SW, 806000-004 (pg 84).
- 5. An iFR cut-point of 0.89 matches best with an FFR ischemic cut-point of 0.80 with a specificity of 87.8% and sensitivity of 73.0%. (iFR Operator’s Manual 505-0101.23)
- 6. N Engl J Med. 2012;367(11):991-1001.
- 7. Witzenbichler B et al. Relationship Between Intravascular Ultrasound Guidance and Clinical Outcomes After Drug-Eluting Stents: The ADAPT-DES Study. Circulation 2014 Jan: 129,4;463-470
- 8. Ahn et al. Meta-Analysis of Outcomes After Intravascular Ultrasound Guided Versus Angiography-Guided Drug-Eluting Stent Implantation in 26,503 Patients Enrolled in Three Randomized Trials and 14 Observational Studies. Am J Cardiol 2014; 113:1338-1347
- 9. Nair A, Margolis M, Kuban B, Vince D. Automated Coronary Plaque Characterisation with Intravascular Ultrasound Backscatter: Ex Vivo Validation. EuroIntervention. 2007; 3: 113-120
- *Safety and efficacy of VH IVUS for use in the characterization of vascular lesions and tissue types has not been established
- Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- Always read the label and follow the directions for use.
- Philips medical devices should only be used by physicians and teams trained in interventional techniques, including training in the use of this device.
- Philips reserves the right to change product specifications without prior notification.
- ©2025 Koniklijke Philips N.V. All rights reserved. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.