The authorization by the FDA for digital pathology in the US, the world’s largest healthcare market, constitutes a watershed moment for the healthcare industry.”
Russ Granzow, General Manager,
Philips Digital Pathology Solutions
Pathologists study disease - by examining cells and tissue sample – and work together in a multidisciplinary team of doctors, scientists, nurses and healthcare professionals to diagnose, treat and prevent illness [1]. Their work underpins every aspect of patient care, from diagnostic testing and treatment advice to the use of cutting-edge genetic technologies. Pathology was until recently one of the last bastions of medicine not to have been digitized.
A historic event came in April 2017 when the U.S. Food and Drug Administration(FDA) permitted marketing of the Philips IntelliSite Pathology Solution for primary diagnostic use in the US. This is the first and currently only time the FDA has permitted the marketing of a digital pathology system for primary diagnosis in the US. From 2014 onwards, Philips IntelliSite has been marketed for diagnostic use in other regions in the world including Europe, Canada, Middle East, Australia and Singapore.
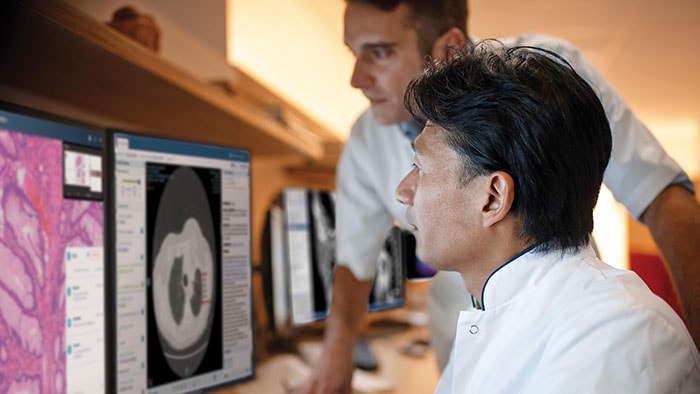
The authorization by the FDA for digital pathology in the US, the world’s largest healthcare market, constitutes a watershed moment for the healthcare industry. Pathologists work in laboratories, in clinics and on hospital wards. Digital pathology enables them to view and diagnose digital images of surgical pathology slides prepared from biopsied and resectioned tissue. It means we can leverage all the benefits that result when images are digitized, including the reporting and billing of diagnostic results.
Rather than looking directly at a tissue sample mounted on a glass slide under a conventional light microscope, the Philips IntelliSite Pathology Solution enables pathologists to read and share tissue slides digitally in order to make diagnoses. It could help make critical health information available to patients and healthcare professionals faster and at lower cost.

A giant leap forward
Incidences of chronic disease are rising around the world, placing immediate demand on pathology. For example, new cancer cases will rise 70% from 14 million in 2012 to 22 million per year in the next two decades [2]. Delays in decision making in pathology account for 41% of delays in cancer diagnosis [3].
Before digital pathology, if second opinions were needed, boxes with glass slides had to be shipped to other specialists, which could take a long time. It also carried a high cost and risk because part of the material could get broken or lost leaving patients and clinicians waiting for the diagnosis.

Increase in digital innovation
Hospitals will have an easier path to realizing a return on investment from their use of a digital pathology system, because pathologists can now charge and get reimbursed.”
ROI was already beginning to become attractive just from the clinical workflow improvements – you load the system, close the door, and there is no more human interaction after that. This could allow high throughput for volume pathology and networked labs with the same or a better level of quality.

The ultimate aim of digitization is that all sorts of algorithms – such as image analysis to aid the pathologist in making better and faster decisions – will be developed and become clinically available. Ultimately, this will create even more opportunities for digital innovation – using artificial intelligence for computational pathology and other innovative technologies – that could support increased accuracies, predictive health management or ultimately potentially improve patient outcomes. [1] The Royal College of Pathologists
Digitizing pathology is indeed taking a giant leap forward and the FDA authorization truly constitutes a watershed moment for the healthcare industry. This achievement reinforces Philips’ leadership in digital pathology, a solution that is central to the diagnosis of complex diseases such as cancer. Now healthcare can reap all the benefits that result when images are digitized, giving clinicians the tools they need to be more effective in this new world of healthcare and making critical health information available faster to healthcare providers and patients.
[2] World Health Organization – Cancer Fact Sheet 2017 and World Cancer Report 2014
About Innovation Matters
Innovation Matters delivers news, opinions and features about healthcare, and is focused on the professionals who work within the industry, as well as Philips as a cutting-edge health technology organization. From interviews with industry giants to how-to guides and features powered by Philips data, our goal is to deliver interesting, educational and entertaining content to empower and inspire all those who work in healthcare or related industries.
You are about to visit a Philips global content page
ContinueShare on social media
Topics
Author

Russ Granzow
General Manager, Philips Digital Pathology Solutions Graduated in Biochemistry. Has a strong track record in deal making, acquisitions and the creation of strategic partnerships in the areas of life science, diagnostics, pharmaceuticals and consumer health. Has significant start up experience, but has also worked with multinationals and as a VC.
Russ began in pharmaceutical research. Was founder of BIAcore (Proteomics) where he led commercial efforts and product development IPO’d in 1996. Founder of Orchid Bioscience, a pharmacogenomics/personalized medicine company IPO’d in 2000. Built up new venture businesses in Philips over the last 9 years. Currently works in the USA and the Netherlands.
Follow me on